When you hear the word "generic," you probably think of cheap pills that work just like the brand-name version. But when it comes to biologics - complex drugs made from living cells - there’s no such thing as a true generic. Instead, there are biosimilars. And while they’re not exact copies, they can save patients, employers, and the healthcare system a lot of money. The question isn’t whether they work - they do. It’s why, after nearly a decade on the market, most biologic spending still goes to the original brands.
What Exactly Are Biosimilars?
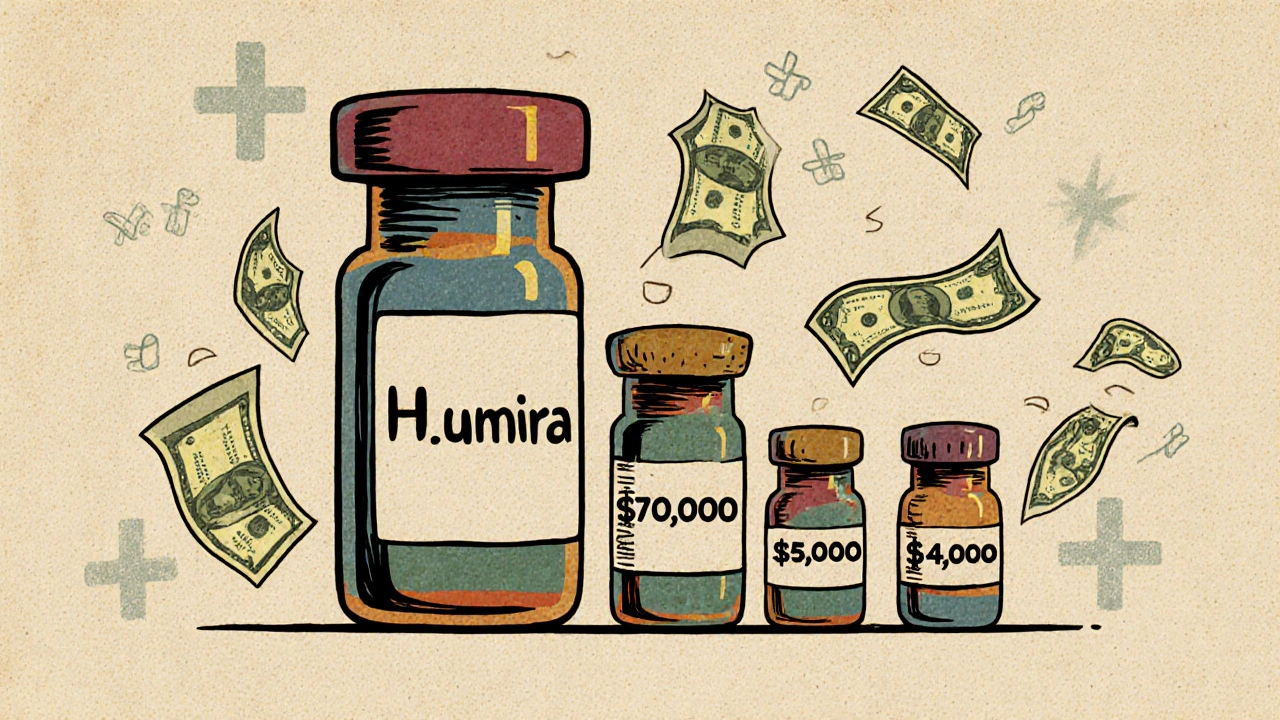
Biosimilars are not like generic pills. Generic drugs are chemically identical to their brand-name counterparts. A 10mg tablet of generic lisinopril is the same molecule, made the same way, every time. Biosimilars are different. They’re made from living organisms - cells, proteins, sometimes even bacteria or yeast. That means even tiny changes in the manufacturing process can affect the final product. So instead of being identical, biosimilars are "highly similar" to the original biologic, with no clinically meaningful differences in safety or effectiveness. The FDA approved the first biosimilar in 2015. Since then, more than a dozen have entered the U.S. market. But while generics can cut prices by 80-90%, biosimilars typically save 15-35% off the list price. That might sound low - until you realize what you’re saving on. Biologics like Humira, Enbrel, and Stelara cost tens of thousands of dollars per year. Even a 20% discount on a $20,000 drug saves $4,000 per patient annually.How Much Do Biosimilars Actually Save?
The savings aren’t just theoretical. In 2024 alone, biosimilars saved the U.S. healthcare system $20.2 billion, according to the Association for Accessible Medicines and IQVIA. Since 2015, that total has reached $56.2 billion. But the real story is in the big-ticket drugs. Take Humira (adalimumab). For years, it was the top-selling drug in the world, costing over $70,000 per patient annually. When the first biosimilars launched in January 2023, list prices dropped by up to 85%. That’s not a small discount - it’s a seismic shift. But here’s the catch: the actual price patients pay isn’t always that low. That’s because drug pricing in the U.S. is a maze of list prices, rebates, and negotiated discounts. The manufacturer of Humira still offers big rebates to pharmacy benefit managers (PBMs), which can make the net price competitive with biosimilars - even if the list price is higher. For Stelara (ustekinumab), the situation is clearer. Nine biosimilars entered the market by July 2025, with list prices as much as 90% below the original. In Medicare Part B, prices for both the originator and biosimilars have steadily dropped after each new entrant. The more biosimilars that come in, the harder manufacturers have to push to keep their prices high.
Why Don’t Biosimilars Save More?
If biosimilars are cheaper and just as safe, why do originator biologics still control 98.9% of biologic spending in 2023? The answer isn’t about science - it’s about money and market structure. First, there’s the rebate trap. Originator companies pay huge rebates to PBMs and insurers to keep their drugs on formularies. These rebates are hidden from patients and even doctors. So even if a biosimilar’s list price is 30% lower, the net price after rebates might be nearly the same. That makes it hard for plans to justify switching. Second, there’s inertia. Doctors are trained to prescribe what they know. Patients are told, "This is the one that works." Many don’t know biosimilars exist - or think they’re "second-rate." A 2025 survey by CSRxP found that 42% of patients were unsure if biosimilars were as safe as the original. Education is still lagging. Third, the U.S. has a massive "biosimilar void." Out of the 118 biologics expected to lose patent protection in the next 10 years, only 12 have biosimilars in development. That means 90% of future biologic spending could remain locked into high-priced originators. In the EU, 73% of high-sales biologics have biosimilars in the pipeline. In the U.S., it’s 23%.Real-World Savings: Patients, Employers, and Systems
The savings aren’t just numbers on a balance sheet. They’re real for people. - Patients: In commercial insurance markets, out-of-pocket costs for biosimilars are 23% lower than for originators. For some, that means dropping from $1,200 a month to $920 - a difference that can make or break whether someone fills their prescription. - Employers: A single employer switching all employees from Humira to a biosimilar can save an average of $1.53 million per year. Across all self-insured U.S. companies, switching just two biologics to biosimilars could save $1.4 billion annually. - Healthcare systems: Every dollar saved on biologics is a dollar that can go toward cancer drugs, mental health services, or diabetes care. Studies show that after biosimilars enter the market, countries reinvest savings into other high-cost therapies. In Norway, biosimilars captured 86% of the market for certain biologics within three years. The U.S. is nowhere near that.
How to Get More Savings - and Why It’s Not Happening Faster
The tools to unlock more savings exist. Employers and health plans can:- Make biosimilars the first-line option on their formularies
- Require patients to try a biosimilar before approving the originator (step therapy)
- Negotiate contracts that reward biosimilar use, not rebates
- Train doctors and educate patients on biosimilar safety and efficacy
The Big Picture: Biosimilars Are a Tool - Not a Magic Bullet
Biosimilars are one of the most promising tools we have to control the cost of modern medicine. They’re not generics. They’re not perfect. But they’re safe, effective, and dramatically cheaper than the originals - when they’re allowed to compete. The $234 billion in potential savings over the next decade isn’t a guess. It’s a calculation based on what’s already happened with Humira, Stelara, and others. The problem isn’t the science. It’s the system. Until we fix the rebate system, stop letting manufacturers block competition, and invest in building a stronger biosimilar pipeline, those savings will stay out of reach. The next time you hear about a new biologic costing $100,000 a year, ask: Is there a biosimilar? If not, why not? Because the answer might be the difference between someone getting treatment - or not.Are biosimilars as safe and effective as the original biologics?
Yes. Biosimilars must meet strict FDA standards to prove they have no clinically meaningful differences in safety, purity, or potency compared to the original biologic. Over 3.3 billion days of patient therapy have been recorded with biosimilars since 2015, with no unique safety concerns identified. Studies published in JAMA Network Open and Frontiers in Pharmacology confirm their effectiveness matches the originator in real-world use.
Why don’t biosimilars save more money than they do?
The main barrier is the U.S. rebate system. Originator drug makers pay large rebates to pharmacy benefit managers (PBMs) and insurers, which can make the net price of the original drug competitive with biosimilars - even when the list price is much higher. This hides the true cost difference from plans and patients. Without transparency in pricing, switching doesn’t happen, even when biosimilars are cheaper on paper.
How do biosimilar savings compare to generic drug savings?
Generics typically save 80-90% off brand-name prices because they’re chemically identical and cheaper to make. Biosimilars, made from living cells, are far more complex to produce. Their savings are smaller - usually 15-35% off list price - but still massive in dollar terms because biologics cost so much. For example, a 30% discount on a $70,000 drug saves $21,000 per patient per year.
Why aren’t there more biosimilars on the market?
Only 12 of the 118 biologics set to lose patent protection in the next decade have biosimilars in development. That’s because developing a biosimilar is expensive, complex, and legally risky. Originator companies use patents, litigation, and exclusivity deals to delay competition. In contrast, the EU has biosimilars in development for 73% of high-sales biologics - compared to just 23% in the U.S.
Can switching to a biosimilar reduce my out-of-pocket costs?
Yes. In commercial insurance markets, patients using biosimilars pay, on average, 23% less out of pocket than those on the originator biologic. For some drugs, like Humira, out-of-pocket savings can reach 45%. If your plan offers a biosimilar option, asking your doctor or pharmacist about switching could lower your monthly costs significantly.
What’s the "biosimilar void," and why does it matter?
The "biosimilar void" refers to the fact that 90% of biologics losing patent protection in the next 10 years have no biosimilar in development. That means $234 billion in potential savings could be lost. Without new biosimilars entering the market, drug prices will stay high, patients will pay more, and healthcare systems won’t be able to reinvest savings into other critical treatments.




Laurie Sala
November 23, 2025 AT 01:10Why does it feel like we’re all just screaming into a void? Biosimilars save billions, but my co-pay’s still $1,200 a month? Someone’s making bank, and it ain’t me. I’ve been on Humira for six years-I’ve seen the price drop on paper, but my wallet? Still bleeding. Why? Because the system’s rigged. And nobody’s talking about the real villains.
Lisa Detanna
November 24, 2025 AT 11:10Let’s be real-this isn’t about science. It’s about power. The pharma giants built empires on these drugs, and they’re not letting go without a fight. But here’s the thing: patients aren’t dumb. We know when we’re being played. And when we finally wake up and demand transparency, this whole house of cards is gonna collapse. The savings are there. We just need the guts to ask for them.
Demi-Louise Brown
November 24, 2025 AT 13:10The data is clear. Biosimilars are safe, effective, and cost-effective. The barriers are systemic, not scientific. Formulary design, rebate structures, and provider education are the key leverage points. Without coordinated policy and transparent pricing, market forces alone will not drive adoption. Structural reform is necessary to align incentives with patient outcomes.
Matthew Mahar
November 24, 2025 AT 19:40ok so i just found out my dr gave me a biosimilar last month and i had no idea?? like… i thought i was still on humira?? and now i’m like… wait i saved like 400 bucks a month?? why wasn’t i told this?? this is wild. someone needs to make a billboard or something. this is life changing and no one’s talking about it
John Mackaill
November 26, 2025 AT 03:58I’ve worked in UK healthcare for 20 years. We’ve had biosimilars since 2007. The savings? Massive. The uptake? Over 80% in most cases. Why? Because the NHS doesn’t play games with rebates. They negotiate directly. No middlemen. No hidden contracts. Patients get the lower price. Simple. The U.S. system is broken-not the science.
Adrian Rios
November 27, 2025 AT 06:22Think about this: every time a doctor prescribes the originator biologic without even considering the biosimilar, they’re not just choosing a drug-they’re choosing a $20,000-a-year expense over a $14,000 one. That’s a $6,000 difference per person. Multiply that by thousands of patients. Multiply that by employers, insurers, Medicare. That’s not just money-it’s opportunity cost. We’re denying cancer patients access to treatment because we’re too lazy to change a formulary or educate a clinician. And we wonder why healthcare is unsustainable.
Casper van Hoof
November 28, 2025 AT 14:00The paradox of biosimilars lies in their very nature: they are scientifically equivalent, yet economically marginalized. The market does not reward efficacy-it rewards inertia, opacity, and contractual entrenchment. The tragedy is not that biosimilars are underutilized, but that their potential is recognized by experts and ignored by institutions. This is not a failure of innovation; it is a failure of moral imagination.
Richard Wöhrl
November 29, 2025 AT 20:21Just checked my last prescription-switched to adalimumab biosimilar last month. Out-of-pocket dropped from $1,180 to $640. That’s $6,480 saved per year. I called my PBM. They said, 'We didn’t notify you because the originator still has a rebate.' So… they’re hiding the savings? I’m not mad-I’m just… shocked. This isn’t healthcare. It’s a financial puzzle where the patient is the last piece to be placed. And we’re all supposed to be grateful when we finally fit?
Brandy Walley
November 29, 2025 AT 21:38