
When your hand starts shaking for no reason - not from caffeine, not from nerves, but when you’re just sitting still - it’s easy to brush it off. Maybe it’s fatigue. Maybe it’s aging. But if that tremor won’t go away, and your movements feel slow, your muscles stiff like they’re wrapped in rubber bands, it might be something deeper. For more than 10 million people worldwide, this isn’t just a passing quirk. It’s Parkinson’s disease.
What’s Really Happening in the Brain?
Parkinson’s isn’t just about shaking. At its core, it’s a breakdown in the brain’s ability to control movement. A group of nerve cells in a region called the substantia nigra, which normally produce dopamine, start dying off. By the time someone notices tremors or stiffness, they’ve already lost 60 to 80% of those dopamine-producing cells. Dopamine isn’t just a mood chemical - it’s the brain’s traffic controller for movement. Without enough of it, signals between the brain and muscles get scrambled. That’s why simple actions like buttoning a shirt, writing a signature, or even standing up from a chair become difficult.The tremor most people associate with Parkinson’s is called a resting tremor. It’s not when you’re moving - it’s when you’re not. Picture a thumb and forefinger rubbing together like you’re rolling a pill. That’s the classic ‘pill-rolling tremor.’ It usually starts on one side of the body, often in the hand or arm, and fades when you reach for a cup or walk around. Stress, tiredness, or strong emotions can make it worse. About 80% of people with Parkinson’s experience this kind of tremor, according to Yale Medicine.
Stiffness: More Than Just Tight Muscles
If you’ve ever felt like your limbs are stuck in molasses, even when you’re not trying to move, you’ve felt what Parkinson’s stiffness feels like. Doctors call it rigidity. Unlike regular muscle tension from exercise or stress, this stiffness doesn’t go away with rest. It’s constant. When a doctor moves your arm or leg during an exam, they might feel a ratchet-like resistance - known as cogwheel rigidity - or a smooth, uniform tightness called lead-pipe rigidity.This isn’t just annoying. It’s painful. Many people develop muscle cramps called dystonia, especially in the feet or neck. Simple tasks become harder: writing becomes tiny and cramped, buttons are impossible to fasten, and tying shoelaces? Forget it. Parkinson’s UK found that 73% of patients struggle with these daily activities within the first three years of diagnosis. The stiffness doesn’t just affect movement - it changes posture, making people hunch forward, which increases the risk of falls.
Dopamine Replacement: The Lifeline That Can’t Cure
There’s no cure for Parkinson’s. But there is a treatment that turns the lights back on - temporarily - for most people: dopamine replacement.The gold standard is levodopa, often combined with carbidopa. Levodopa is a chemical the body turns into dopamine. Carbidopa stops it from breaking down before it reaches the brain, so less of it is wasted and fewer side effects like nausea occur. This combo, first approved in 1970, is still the most effective treatment we have. Around 75% of patients see major improvement in movement within 30 to 60 minutes of taking it.
But here’s the catch: it doesn’t last. After 5 to 10 years, many people start experiencing wearing-off - where the medicine’s effects fade before the next dose. Then come the on-off fluctuations: one moment you’re moving freely, the next you’re frozen. And then there’s dyskinesia - involuntary, dance-like movements that can be as disabling as the original symptoms. About 40 to 50% of long-term users develop these complications, according to the Journal of Parkinson’s Disease.
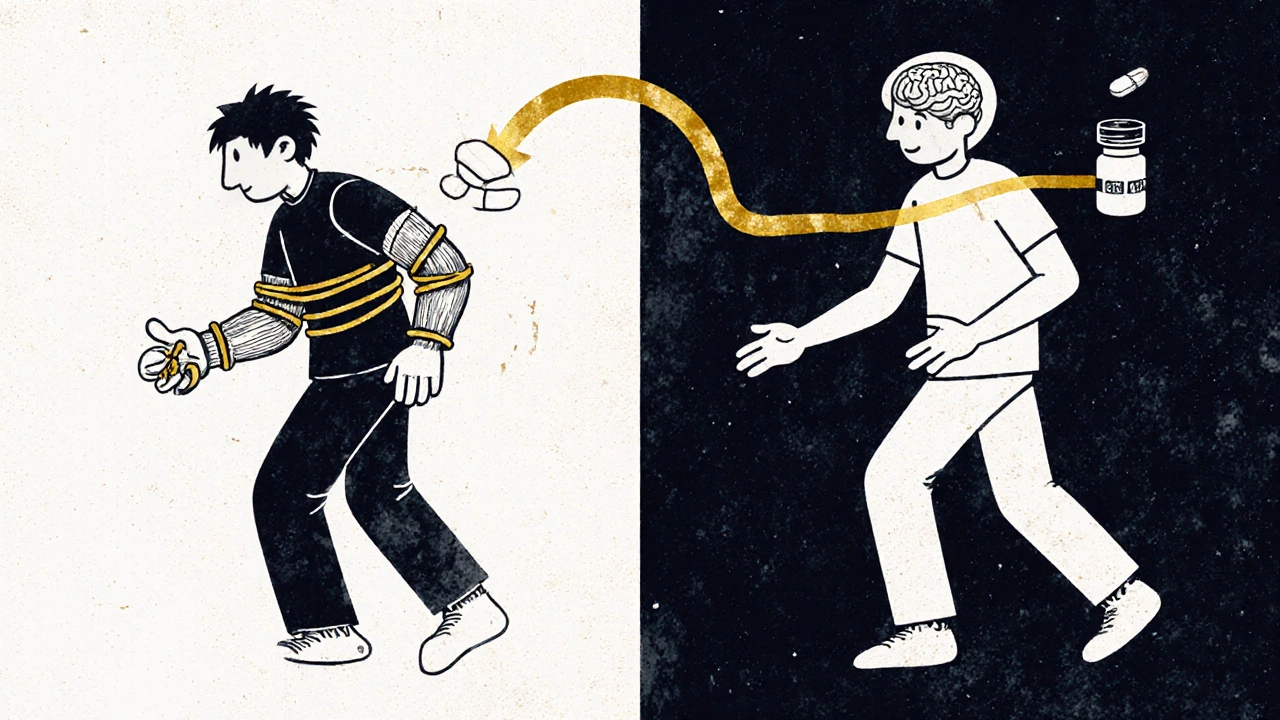
Alternatives and Add-Ons
Not everyone starts with levodopa. For younger patients, doctors often begin with dopamine agonists like pramipexole or ropinirole. These drugs mimic dopamine by directly stimulating its receptors. They’re not as strong as levodopa - about 30 to 50% as effective - but they carry a lower risk of early dyskinesia. Many people use them early to delay levodopa, then add levodopa later as symptoms worsen.The Cleveland Clinic reports that 60% of patients eventually need both. It’s not about choosing one or the other - it’s about layering treatments as the disease changes. Newer forms of levodopa, like Rytary (an extended-release version), help smooth out the highs and lows, letting some people take just two doses a day instead of four or five. But Rytary costs about $5,800 a year. The generic immediate-release version? Around $600.
The Protein Problem and Other Practical Hurdles
Taking dopamine medicine sounds simple - until you realize how many rules come with it. Levodopa competes with amino acids from protein for absorption in the gut. That means if you eat a big steak or a bowl of beans right before your pill, it might not work. Many patients learn to take their medication 30 to 60 minutes before meals or wait an hour after eating. This isn’t just a suggestion - it’s often the difference between moving and being stuck.Timing becomes everything. One patient on the Parkinson’s Foundation forum, ‘ParkinDad,’ shared that after eight years on levodopa, his ‘on’ time dropped from six hours per dose to just two or three. Another, ‘SilverLining2022,’ found pramipexole kept symptoms under control for five years with few side effects. There’s no one-size-fits-all.
Managing meds becomes a full-time job. The Parkinson’s Foundation says 78% of patients need help from caregivers to track doses, meals, and side effects. What starts as 15 minutes a day managing pills can balloon to 45 minutes as the disease progresses. And even then, only 35% of community neurologists follow all the recommended guidelines for dosing and timing, according to the PD GENEration study.

What’s Next? Beyond Pills
Researchers aren’t giving up. In 2018, the FDA approved Inbrija - a levodopa inhaler that kicks in within 10 minutes for sudden ‘off’ episodes. It’s expensive - about $3,700 a month - but for someone frozen mid-step, it’s a lifeline.More promising are continuous delivery systems. The RESTORE-1 trial in 2022 tested a subcutaneous infusion of Foslevodopa/foscarbidopa. Patients got 2.5 more ‘on’ hours per day compared to oral meds. No more waiting for a pill to kick in. No more unpredictable crashes. It’s still not widely available, but it’s a glimpse of what’s coming.
Gene therapies and biomarkers are also in the works. The Michael J. Fox Foundation’s Parkinson’s Progression Markers Initiative has spent over $115 million since 2010 trying to find biological signals that predict who will respond best to which drug. Early data suggests genes like COMT and MAO-B affect how your body breaks down dopamine - meaning someday, your DNA could guide your treatment plan.
It’s Not Just About Movement
Parkinson’s doesn’t stop at tremors and stiffness. Many people struggle with sleep problems, depression, constipation, and loss of smell years before movement symptoms show up. But for most, the motor symptoms are what make daily life unrecognizable. The goal of dopamine replacement isn’t to cure - it’s to buy time. Time to walk the dog. Time to hold a grandchild’s hand. Time to eat without help.There’s no perfect solution. Levodopa works wonders - until it doesn’t. Agonists help delay complications - but they’re weaker. New delivery systems are expensive. And the emotional toll of watching your body change, of needing help for things you once did alone, is something no pill can fix.
What matters most is personalized care. Starting low, going slow. Listening to what your body tells you. Adjusting not just the dose, but the timing, the meals, the support system. Parkinson’s doesn’t follow a script. Neither should your treatment.
Can dopamine replacement cure Parkinson’s disease?
No. Dopamine replacement, like levodopa, helps manage symptoms by replacing the dopamine the brain no longer makes. It doesn’t stop the death of nerve cells or reverse damage. It’s a treatment, not a cure. People often feel dramatically better at first - but over time, the disease continues to progress, and the medication’s effectiveness can decline or cause new problems like dyskinesia.
Why does levodopa stop working after a few years?
As Parkinson’s progresses, the brain loses more dopamine-producing cells. This means there are fewer places for levodopa to be converted into dopamine. The brain also becomes less able to store and release dopamine smoothly. This leads to ‘wearing-off’ - where the drug’s effect fades before the next dose - and ‘on-off’ fluctuations, where movement suddenly switches between free and frozen. Long-term use can also trigger dyskinesias, which are involuntary movements caused by too much dopamine in certain brain areas.
Is it better to start dopamine therapy early or wait?
There’s no one-size-fits-all answer. Earlier guidelines warned that starting levodopa too soon might lead to earlier side effects. But research now shows levodopa doesn’t speed up disease progression. The current recommendation is to start when symptoms begin to affect quality of life. For younger patients (under 60), doctors often delay levodopa and use dopamine agonists first to postpone motor complications. For older patients or those with severe symptoms, levodopa is started sooner because its benefits outweigh the risks.
Can diet affect how well Parkinson’s medication works?
Yes. High-protein meals can block levodopa from being absorbed in the gut because they compete for the same transport system. Many patients find that taking levodopa 30 to 60 minutes before meals improves its effectiveness. Some doctors recommend spreading protein intake evenly across the day or having a low-protein dinner to help nighttime medication work better. It’s not about cutting protein entirely - it’s about timing.
What are the most common side effects of dopamine replacement?
Nausea and dizziness are common at first, especially with levodopa. Carbidopa helps reduce these. Long-term side effects include dyskinesias (involuntary movements), ‘on-off’ fluctuations, and sometimes hallucinations or impulse control issues like gambling or increased shopping. About 63% of users report nausea, 42% dizziness, and 38% dyskinesias, according to PatientsLikeMe. These side effects often become more noticeable as the dose increases over time.
Are there non-medication ways to manage Parkinson’s symptoms?
Yes. Exercise is one of the most powerful tools. Studies show that regular aerobic activity, tai chi, dance, and strength training can improve balance, stiffness, and even slow the decline in mobility. Physical therapy helps with posture and gait. Speech therapy addresses soft speech and swallowing issues. A healthy diet supports overall well-being, and mental health support helps with depression and anxiety - both common in Parkinson’s. Medication helps with movement, but lifestyle changes help you live better with it.




robert cardy solano
November 21, 2025 AT 00:37Been watching my dad go through this for 8 years. Levodopa was a miracle at first - then it became a clock you have to wind every 3 hours. He eats protein at dinner like normal, and by 10pm he’s stuck on the couch like a statue. No one tells you about the meal timing thing until you’re already late for your own life.
swatantra kumar
November 21, 2025 AT 16:21Man, this hits different. I’ve got a cousin in Delhi with PD and he’s on generics - $50/month. Meanwhile, his cousin in Texas is on Rytary and crying over insurance denials. It’s not a disease, it’s a tax on being sick in America 🤦♂️💸
Brianna Groleau
November 22, 2025 AT 06:27I remember when my mom first started shaking. We thought it was anxiety. Then she couldn’t button her coat. Then she couldn’t stand up without help. Levodopa gave her back her dignity - even if it was just for a few hours. I don’t care if it’s not a cure. It’s the difference between watching someone fade… and watching them live. That’s worth everything.
Bill Camp
November 22, 2025 AT 11:08Let’s be real - Big Pharma is laughing all the way to the bank. Levodopa’s been around since the 70s. They didn’t invent a better drug. They just made it more expensive and called it ‘innovation.’ Rytary? $5,800 a year? That’s not medicine - that’s extortion dressed in a lab coat.
Dave Wooldridge
November 22, 2025 AT 17:05Did you know the CDC quietly admitted in 2021 that glyphosate exposure is linked to dopamine neuron death? And they still let it in our food? This isn’t ‘natural aging’ - it’s chemical warfare. They don’t want you to know. That’s why they call it ‘idiopathic.’
Cinkoon Marketing
November 23, 2025 AT 01:57My neurologist said I should wait to start levodopa until I really need it. But then I tried to pick up my coffee mug and it spilled everywhere. I was 58. I didn’t want to be the person who couldn’t drink coffee without help. So I started. And yeah, I got dyskinesia. But at least I could still hug my grandkids. Worth it.
Matthew McCraney
November 23, 2025 AT 06:19you guys are all so naive. dopamine replacement? lol. its all a psyop. the real cure is magnetic field therapy and turmeric juice. they dont want you to know because the pharma giants make billions off your suffering. i saw a video on yt where a guy in russia reversed parkinsons with a potato and a magnet. its true. i swear on my moms grave.
Nick Naylor
November 23, 2025 AT 17:39According to the American Neurological Association’s 2023 Position Paper on Motor Degenerative Disorders (ANAP-MDD-2023-04), the pharmacokinetic variability of levodopa absorption is directly correlated with gastric pH levels, which are significantly modulated by dietary protein intake - specifically, branched-chain amino acids (BCAAs) such as leucine, isoleucine, and valine - which compete with levodopa for the L-type amino acid transporter (LAT1) at the blood-brain barrier. Consequently, the clinical recommendation is not merely ‘timing,’ but a structured, time-restricted, low-BCAA dietary protocol administered in conjunction with medication schedules - a protocol followed by fewer than 12% of U.S. neurology clinics, per the PD GENEration Study. This is not negligence - it is systemic failure.
Pawan Jamwal
November 25, 2025 AT 07:21India has over 1.2 million PD patients and less than 300 neurologists who specialize in movement disorders. We use generics because we have to. My uncle takes levodopa with a banana - because he can’t afford to wait. He walks 5 km every morning to the clinic. No fancy inhalers. No infusions. Just grit. We don’t need American prices to feel human.
Sarah Swiatek
November 27, 2025 AT 05:36I used to be a nurse on the neuro floor. I saw people on levodopa go from wheelchair to walking again - then, five years later, they’d be dancing uncontrollably while crying because they couldn’t hold a spoon. It’s beautiful and tragic. The real tragedy isn’t the disease - it’s that we treat symptoms like they’re the enemy, instead of listening to the person behind them. I miss the days when we just sat with patients and asked, ‘What do you need today?’ Not ‘What dose are you on?’
rob lafata
November 28, 2025 AT 10:48You people are pathetic. You treat this like a medical problem. It’s a moral failure. You let your bodies rot because you’re too lazy to move, too weak to fight, too addicted to convenience. You take pills like candy and then blame Big Pharma. You don’t need levodopa. You need a boot up your ass and a 5AM run. I’ve seen people with worse than PD hike mountains. You just want an excuse to quit.
Rusty Thomas
November 29, 2025 AT 01:53My sister got diagnosed at 42. She started dancing - ballroom, salsa, everything. Said it made her feel alive. Now she’s in a wheelchair. But she still dances in her head. I cried when I saw her trying to tap her foot to music while her body wouldn’t move. That’s not a disease. That’s a love story.
Lemmy Coco
November 30, 2025 AT 23:46just wanted to say i started on pramipexole 5 years ago and it worked great until last month when i started getting weird urges to buy stuff online… like 3000$ worth of tools i dont need. i think i have impulse control issue now. also my spelling is bad sorry
serge jane
December 1, 2025 AT 02:06There’s something profoundly human about the way we cling to control - we want a cure, a pill, a timeline, a fix. But Parkinson’s doesn’t care about our calendars or our insurance plans. It doesn’t care if you’re rich or poor, young or old. It just keeps moving. And in that silence, in the space between the tremor and the stillness, maybe we’re not losing our movement - maybe we’re learning how to be still in a world that never stops. The pills buy time. But the quiet? The quiet teaches us how to live inside the brokenness. And maybe that’s the only cure we ever really needed.