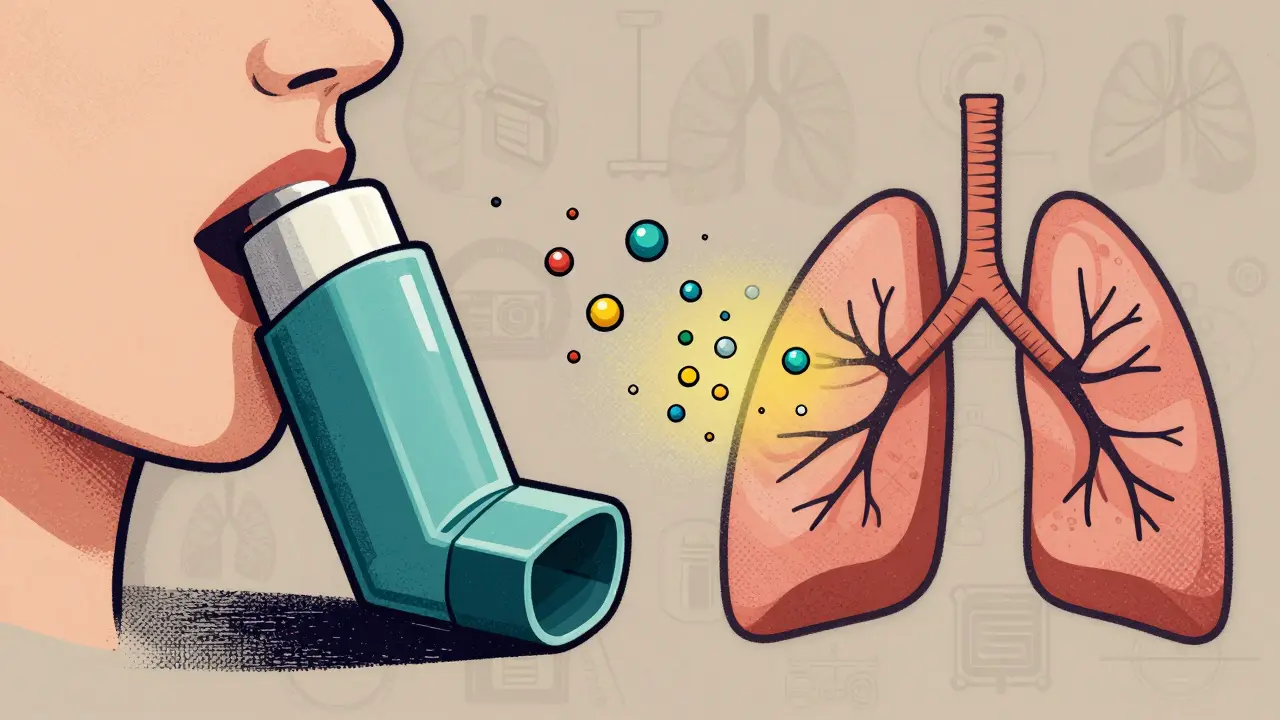
Why bioequivalence matters more than you think
When you pick up a generic inhaler, patch, or injection, you assume it works just like the brand-name version. But that’s not always guaranteed. Unlike pills, these delivery systems don’t just dissolve in your stomach and get absorbed into your blood. They’re designed to deliver drugs in very specific ways-to your lungs, through your skin, or directly into your bloodstream. And even tiny differences in how they work can change how well they treat your condition.
That’s where bioequivalence comes in. It’s not just a buzzword. It’s the scientific proof that a generic version delivers the same amount of medicine, at the same speed, to the right place in your body. For oral drugs, this is relatively simple: measure blood levels over time. But for inhalers, patches, and injections? It’s a whole different ballgame.
Inhalers: It’s not just about the drug
Take an asthma inhaler. You press the canister, breathe in, and the medicine lands in your lungs. Sounds simple. But the real trick isn’t the drug-it’s the device. The size of the particles, how they’re sprayed, even the temperature of the plume matters. If the particles are too big, they hit your throat and get swallowed. Too small, and they fly out when you exhale. Either way, you’re not getting the full dose.
The FDA requires inhaler generics to match the reference product in three key ways: particle size (90% between 1 and 5 micrometers), total dose delivered (within 75-125% of the label), and plume geometry (how the spray spreads in the air). In one case, a generic albuterol inhaler was rejected because its plume was 2°C warmer than the brand. That tiny difference changed how the spray behaved in the air, even though the drug amount was identical.
For corticosteroid inhalers-used for long-term control-blood tests don’t cut it. The drug works locally in the lungs. So regulators look at lung function instead. They measure how much your FEV1 (forced expiratory volume in one second) improves after using the inhaler. If the generic doesn’t improve lung function just as well, it’s not approved.
Transdermal patches: Slow and steady wins the race
Patches are designed to release medicine slowly through your skin over hours or days. Think nicotine patches for quitting smoking or fentanyl patches for chronic pain. Because they work over time, the peak blood level (Cmax) doesn’t tell the whole story. What matters is the total amount absorbed over 24 hours (AUC).
The FDA requires patch generics to match the original in three areas: how fast the drug comes out of the patch (in vitro release rate), how well it sticks to your skin (adhesion), and how much drug is left behind after use (residual content). The release rate must be within 10% of the brand at every time point. If it’s too fast, you get a spike in drug levels-risking side effects. Too slow, and you don’t get enough relief.
Unlike oral drugs, the 80-125% bioequivalence range isn’t always applied to Cmax. For highly variable drugs like some pain medications, regulators use a method called reference-scaled average bioequivalence. It’s more flexible, but still strict. One company spent over $30 million and four years developing a generic testosterone patch, only to have it rejected because the adhesive didn’t hold as well in humid conditions.

Injections: When the container matters as much as the drug
Injectables are the toughest. Liposomal doxorubicin, insulin glargine, enoxaparin-these aren’t simple solutions. They’re complex mixtures: nanoparticles, fat bubbles, or long-chain molecules designed to last longer in your body. A generic version can’t just copy the formula. It has to match the physical structure too.
The FDA requires identical particle size (within 10%), polydispersity (how uniform the particles are, must be under 0.2), and zeta potential (electrical charge, within 5mV). For enoxaparin, a blood thinner with a narrow therapeutic window, the acceptable range for AUC and Cmax is tighter: 90-111%. One generic version of Lovenox was pulled from the market after patients reported more clotting events-turns out, the particle size distribution was slightly off.
Even the auto-injector matters. The FDA rejected a generic Bydureon BCise because the injector’s spring mechanism delivered the drug too slowly. The drug was chemically identical, but the delivery speed changed how quickly it entered the bloodstream. That’s not a manufacturing flaw-it’s a bioequivalence failure.
Why approval rates are so low-and why it costs so much
While 78% of standard oral generics get approved, only 38% of inhalers, 52% of patches, and 58% of complex injectables make it through. Why? Because each one needs a multi-layered proof package. You’re not just running one blood test. You’re doing in vitro release tests, particle analysis, skin adhesion studies, lung function measurements, and sometimes even imaging scans to track where the drug goes in the body.
The cost? $25-40 million per product. That’s five times more than a regular generic. The timeline? Three to four years. Compare that to 18-24 months for a pill. And it’s not just the science-it’s the equipment. Cascade impactors for inhalers cost $300,000. Franz diffusion cells for patches? $100,000. Particle analyzers for injectables? Over $200,000.
Only big companies can afford this. Teva, Mylan, and Sandoz control most of the market. Small developers struggle. The FDA has a special program to help small businesses, but only 42 have received support since 2018.
Success stories and failures: Real-world impact
There are wins. Teva’s generic ProAir RespiClick was approved in 2019 after using scintigraphy imaging to prove identical lung deposition. Within 18 months, it captured 12% of the market. Patients got the same relief at a fraction of the cost.
But failures hurt. A generic version of Advair Diskus was turned down in 2019 because the fine particle fraction was 5% lower-even though the total dose was correct. Patients using it had more asthma flare-ups. The FDA didn’t just say “close enough.” They said, “This could harm people.”
And then there’s the biocreep risk. Imagine five generations of generics, each slightly different. Individually, each passes bioequivalence. But over time, the cumulative changes might reduce effectiveness. Experts are now warning that we need long-term monitoring systems to catch this before patients are affected.
What’s next for bioequivalence?
The field is evolving fast. Regulators are moving away from relying only on blood tests. They’re using physiologically-based pharmacokinetic (PBPK) modeling-computer simulations that predict how a drug behaves in the body based on its physical properties. In 2022, 65% of complex generic submissions included PBPK models. That’s up from 22% in 2018.
The EMA now requires patient training materials as part of inhaler approval. If the device is hard to use, even a perfect generic won’t help. The FDA is also drafting new rules for monoclonal antibody injections, which could change how biosimilars are approved.
The goal? More generics. The market for complex generics is expected to hit $112 billion by 2027. But the path is narrow. Every step requires precision, patience, and proof that the medicine doesn’t just look the same-it works the same.
What patients should know
If you’re prescribed a generic inhaler, patch, or injection, ask your pharmacist or doctor: “Has this been proven to work just like the brand?” Don’t assume it’s the same. For complex delivery systems, bioequivalence isn’t automatic. It’s earned through science.
And if your symptoms change after switching to a generic-more wheezing, less pain relief, unexpected side effects-tell your provider. It might not be your body. It might be the delivery system.


Beth Cooper
January 30, 2026 AT 08:28Melissa Cogswell
January 30, 2026 AT 21:40Diana Dougan
February 1, 2026 AT 02:46Bobbi Van Riet
February 1, 2026 AT 17:15Holly Robin
February 3, 2026 AT 05:13Shubham Dixit
February 3, 2026 AT 05:40KATHRYN JOHNSON
February 4, 2026 AT 19:02Blair Kelly
February 5, 2026 AT 22:31Rohit Kumar
February 7, 2026 AT 06:32Lily Steele
February 8, 2026 AT 19:58Eliana Botelho
February 10, 2026 AT 02:19Niamh Trihy
February 10, 2026 AT 15:55Beth Beltway
February 12, 2026 AT 05:34Marc Bains
February 13, 2026 AT 03:16Natasha Plebani
February 14, 2026 AT 21:50