
When you pick up a prescription, you might not notice the difference between the brand-name pill and the generic one sitting in your bag. But behind that switch is a complex, science-backed system called Therapeutic Equivalence Codes-or TE Codes. These codes tell pharmacists exactly which generic drugs can be safely swapped for brand-name versions without changing how well the medicine works. It’s not guesswork. It’s not opinion. It’s a federal standard that saves billions every year and keeps millions of Americans on affordable treatment.
What Are TE Codes and Why Do They Matter?
Therapeutic Equivalence Codes are assigned by the U.S. Food and Drug Administration (FDA) and published in the Approved Drug Products with Therapeutic Equivalence Evaluations, better known as the Orange Book. First introduced in 1984 after the Hatch-Waxman Act, these codes were created to solve a real problem: pharmacists didn’t have clear rules on which generics could be substituted. Some states allowed it, others didn’t. Doctors were confused. Patients got mixed results. The solution? A standardized system where every multi-source drug-meaning any medicine with more than one manufacturer-is evaluated and given a code. That code tells you if the generic is a true substitute. The most common code is AA. That means the generic has the same active ingredient, same strength, same dosage form, and has been proven to work the same way in your body as the brand-name drug. If you see a B code, you don’t substitute. It’s not equivalent. This system isn’t just paperwork. It’s the reason 90% of all prescriptions filled in the U.S. today are generics. And they cost 80-85% less. A 30-day supply of Lipitor (atorvastatin) might run $150 brand-name. The generic? Around $10. That’s not a marketing claim-it’s a TE Code guarantee.How the FDA Decides If a Generic Is Equivalent
The FDA doesn’t just look at the ingredients. They dig deep. To earn an ‘A’ rating, a generic must pass three tests:- Pharmaceutical equivalence: Same active ingredient, same strength, same form (tablet, capsule, injection, etc.), same route of administration.
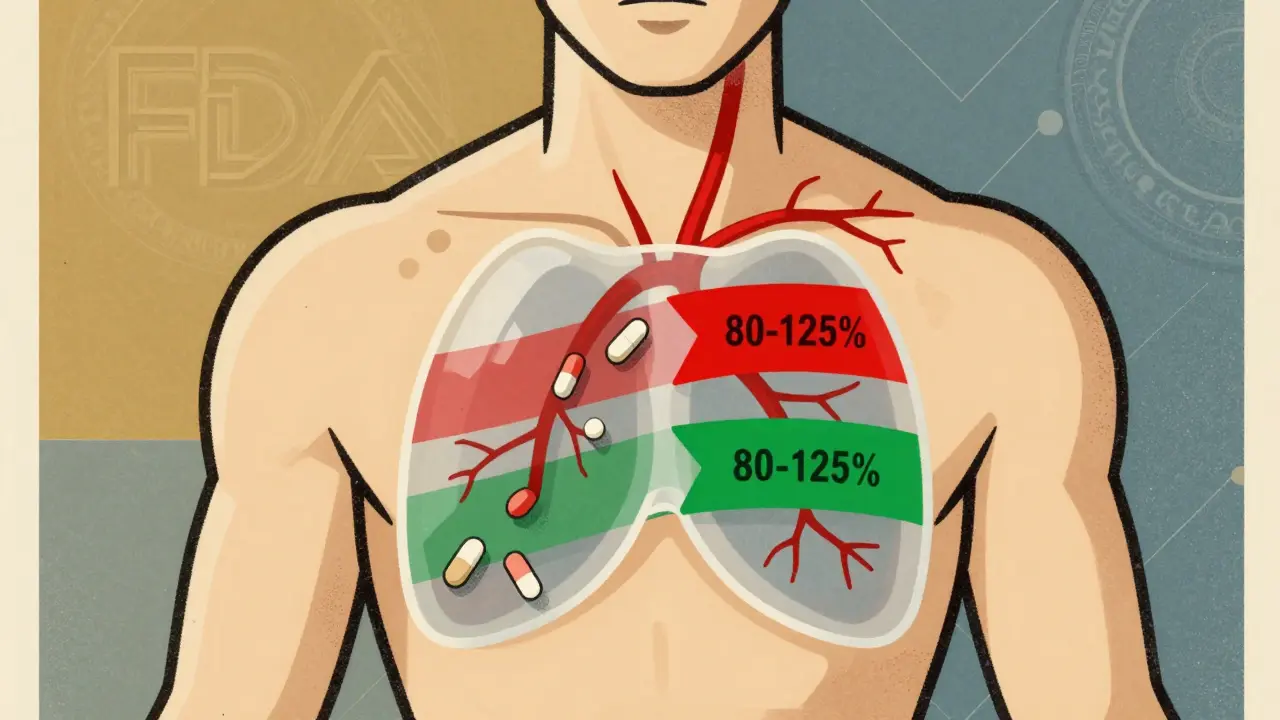
- Bioequivalence: The generic must deliver the same amount of medicine into your bloodstream at the same rate as the brand. That’s measured using blood tests called AUC and Cmax. The FDA requires the generic’s results to fall within 80-125% of the brand’s. That’s not a wide margin-it’s tight.
- Clinical equivalence: No meaningful difference in safety or effectiveness under real-world use. This is where the Orange Book gets its authority. If two drugs have the same TE code, they’re treated as interchangeable in every state.
Understanding the TE Code Letters
The code looks simple-just two or three letters-but each one carries meaning. The first letter is always A or B.- A = Therapeutically equivalent. Safe to substitute.
- B = Not equivalent. Don’t substitute without doctor approval.
- AA = Oral solution, powder for oral solution
- AN = Injectable solution
- AO = Oral solution
- AP = Powder for injection
- AT = Topical cream or ointment
How Pharmacists Use TE Codes Every Day
In a busy pharmacy, a technician pulls up a prescription. The doctor wrote “Lisinopril 10 mg.” The brand is Zestril. But the patient’s insurance only covers generics. The pharmacist checks the Orange Book-online or built into their pharmacy software-and sees the generic has an AP code. That means it’s an approved, equivalent substitute. The pharmacist swaps it. No call to the doctor. No paperwork. Just a quick lookup. That’s the power of TE Codes. According to the National Community Pharmacists Association, 91% of pharmacists feel confident making these substitutions because the system is clear and consistent. It’s not just convenience. It’s compliance. Every state in the U.S. has laws that require pharmacists to substitute generic drugs when the TE Code says it’s allowed. Only if the doctor writes “Dispense as Written” or “Do Not Substitute” does the pharmacist have to give the brand.Where TE Codes Fall Short
Despite their success, TE Codes aren’t perfect. They don’t cover everything. Narrow therapeutic index (NTI) drugs are the biggest concern. These are medicines where even tiny changes in blood levels can cause harm or reduce effectiveness. Warfarin (Coumadin), levothyroxine, and some seizure meds fall into this category. The FDA says TE-rated generics are still safe-but some patients report feeling different after switching, even when lab results look fine. A 2022 study found 12.7% of patients perceived a difference after switching between TE-rated generics. That doesn’t mean the drug didn’t work-it means their body reacted differently to the inactive ingredients, or the timing of absorption shifted slightly. Complex products like inhalers, patches, and topical creams are also tricky. The active ingredient might be the same, but how it’s delivered can vary. A generic inhaler might deliver less medicine to the lungs than the brand, even if blood levels look normal. That’s why the FDA has pulled TE ratings for certain generic budesonide inhalers and is now working on new codes for these products. And TE Codes don’t consider individual patient factors. A 70-year-old with kidney disease might metabolize a drug differently than a 30-year-old. The code doesn’t know that. That’s why doctors still need to be involved in complex cases.

Kristen Russell
January 1, 2026 AT 16:28Love how this breaks down the science without the fluff. I’ve been on generics for years and never knew the FDA had such tight standards-now I actually trust the switch. Thanks for this!
Bryan Anderson
January 3, 2026 AT 15:02Thank you for this thorough overview. The bioequivalence criteria-particularly the 80–125% AUC and Cmax range-are often misunderstood. It’s reassuring to see how rigorously these thresholds are enforced. The Orange Book remains an underappreciated public health asset.
Matthew Hekmatniaz
January 4, 2026 AT 16:56As someone who’s worked in rural pharmacies for over a decade, I’ve seen firsthand how TE codes keep care accessible. We don’t have specialists down the street-so when a patient gets a $10 generic instead of $150 brand, it’s not just savings-it’s adherence. I wish more people knew how much trust we put in these codes daily.
That said, I’ve had patients tell me they ‘feel different’ after switching-even with AA codes. We always document it and check in. It’s not about doubting the system, it’s about honoring individual biology.
Liam George
January 6, 2026 AT 00:53Let’s be real-this whole TE code system is a corporate puppet show. The FDA’s ‘bioequivalence’ standards? A joke. They let generics pass with 80–125% variability? That’s a 45% swing! Meanwhile, Big Pharma owns the labs, the reviewers, and the ‘data.’ You think your levothyroxine switch is safe? Try asking someone who went from Synthroid to a $5 generic and ended up in the ER with atrial fibrillation. The system’s rigged to save money, not lives.
And don’t get me started on how they ‘updated’ the codes for inhalers-after lawsuits, not science. The Orange Book is a PR brochure, not a medical bible. Wake up.
Phoebe McKenzie
January 6, 2026 AT 05:44Wow. Just… wow. This post is a masterclass in how the system is being exploited. You people are so naive. The FDA doesn’t care about you. They care about profits. That ‘AA’ code? It’s a lie. Look at the lawsuits. Look at the recalls. Look at the whistleblowers. People are dying because some lab technician in China got paid $3/hour to fake dissolution tests. And you’re just nodding along like it’s all fine? Shame.
gerard najera
January 6, 2026 AT 15:30Efficiency without empathy is just automation.
Donna Peplinskie
January 7, 2026 AT 13:31This is so helpful-I’ve been trying to explain TE codes to my sister, who’s terrified of generics after her mom had a bad reaction. I’ll send her this. It’s so important to acknowledge the NTI drug concerns, though. I’ve seen people panic when their meds change, even if it’s ‘equivalent.’ A little more compassion in the messaging could go a long way.
Lee M
January 9, 2026 AT 06:56TE codes are the last illusion of control in a broken system. We treat medicine like a spreadsheet. But the body isn’t a lab rat. The 80–125% window? That’s not science-it’s compromise. And now we’re extending it to biosimilars? We’re not advancing health-we’re optimizing cost. The real question isn’t whether they’re equivalent-it’s whether we’ve forgotten what healing even means.