Levodopa Absorption Calculator
How Protein Affects Your Medication
High protein meals compete for the same transporters in your gut that carry levodopa into your bloodstream. This can reduce how much medication reaches your brain.
Enter your protein intake (in grams) to see how it impacts absorption:
Absorption Impact
0% absorption
When doctors prescribe a pill for Parkinson’s disease, the most common name you’ll hear is Carbidopa-Levodopa a fixed‑dose combination that restores dopamine levels in the brain. But why does mixing two chemicals work better than taking either one alone? The answer lies in the drug’s clever use of biology - a step‑by‑step mechanism of action that exploits how our bodies transport and convert molecules. This article breaks down every stage, from swallowing the tablet to feeling the tremor‑free relief.
Key Takeaways
- Carbidopa blocks peripheral conversion of levodopa, letting more reach the brain.
- Levodopa crosses the blood‑brain barrier via active transport and is then turned into dopamine.
- The combination reduces nausea, vomiting, and the need for high doses.
- Understanding the pharmacokinetics helps clinicians fine‑tune dosing schedules.
- Side effects often stem from overstimulation of dopamine pathways.
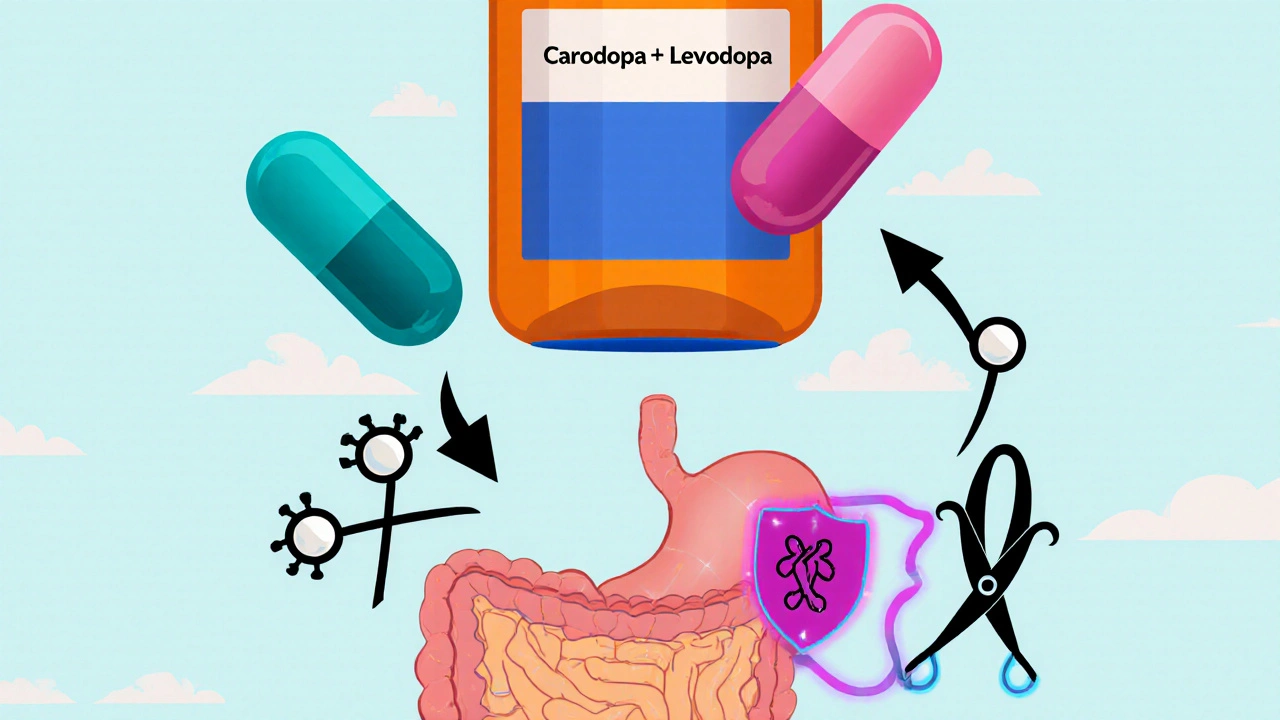
What Is Carbidopa-Levodopa?
Levodopa the metabolic precursor of dopamine was first synthesized in the 1960s and quickly proved effective at easing Parkinson’s motor symptoms. However, when taken by mouth, most of it never reaches the brain because enzymes in the gut and bloodstream convert it into dopamine before it can cross the blood‑brain barrier a tightly regulated membrane that protects the central nervous system. The resulting peripheral dopamine can’t help the brain and instead causes gastrointestinal upset.
Enter Carbidopa a peripheral aromatic L‑amino acid decarboxylase (AADC) inhibitor. Carbidopa does not cross the blood‑brain barrier, but it blocks the enzyme that would otherwise turn levodopa into dopamine outside the brain. By pairing the two, manufacturers created a formulation that delivers more levodopa where it matters while minimizing unwanted peripheral effects.
Why Combine? The Synergy Behind the Duo
Imagine trying to fill a bathtub with a leaky pipe. Levodopa alone is that leaky pipe - it drips away in the gut, and only a small fraction reaches the target. Carbidopa acts like a clamp that stops the leak, allowing the water (levodopa) to flow straight to the tub (brain). This synergy translates into three practical benefits:
- Higher central availability: Up to 85% of the administered levodopa reaches the brain when combined with carbidopa, versus only 10‑15% alone.
- Lower dosing requirements: Patients can achieve symptom control with half the milligram dose, reducing cost and pill burden.
- Fewer peripheral side effects: Nausea, vomiting, and orthostatic hypotension drop dramatically.
These advantages are why the combination remains the first‑line therapy for most Parkinson’s patients.
Crossing the Blood‑Brain Barrier: How Levodopa Gets In
Levodopa is structurally similar to the essential amino acid L‑tyrosine. The brain transports both through the same large neutral amino acid (LNAA) transporter, a protein embedded in the endothelial cells of the blood‑brain barrier. When you eat a protein‑rich meal, competition for this transporter increases, which can blunt levodopa’s absorption. That’s why clinicians often advise taking the medication on an empty stomach or spacing meals 30 minutes before or after dosing.
Once inside the brain, levodopa encounters the enzyme Aromatic L‑Amino Acid Decarboxylase the same enzyme inhibited peripherally by carbidopa, but now active in neuronal tissue. This enzyme removes a carboxyl group, converting levodopa into dopamine directly within dopaminergic neurons.
The Role of Carbidopa: Blocking Peripheral Decarboxylation
Carbidopa’s molecular size and polarity prevent it from slipping through the blood‑brain barrier. In the gut and bloodstream, it binds tightly to AADC, rendering the enzyme inactive. As a result:
- Levodopa stays intact longer, increasing its half‑life from roughly 60 minutes (alone) to 90‑120 minutes when paired.
- Peripheral dopamine production drops, which means less nausea and fewer cardiac side effects.
- The central conversion rate stays high because brain AADC is untouched.
Clinically, this translates into smoother ON periods (when symptoms are controlled) and fewer OFF fluctuations (when symptoms return).
From Levodopa to Dopamine: The Final Step
Inside the basal ganglia, the freshly formed dopamine binds to D1 and D2 receptors. These receptors modulate two parallel pathways that regulate movement:
- D1‑direct pathway: Facilitates movement, reducing rigidity.
- D2‑indirect pathway: Inhibits competing signals, smoothing out tremors.
By restoring dopamine tone, patients experience reduced bradykinesia (slowness), fewer tremors, and improved gait. However, because dopamine also influences mood, behavior, and autonomic functions, some patients may develop dyskinesias (involuntary movements) after long‑term use.
Pharmacokinetics at a Glance
The combined formulation comes in several oral forms: immediate‑release tablets, sustained‑release capsules, and dispersible tablets for those with swallowing difficulties. Here’s a quick snapshot of key parameters:
| Parameter | Immediate‑Release | Sustained‑Release |
|---|---|---|
| Onset of effect | 30-60 minutes | 60-90 minutes |
| Peak plasma concentration | 1-2 hours | 2-4 hours |
| Half‑life | 90-120 minutes | 180-240 minutes |
| Duration of motor benefit | 3-5 hours | 6-8 hours |
Doctors often start with low doses and titrate upward, balancing symptom control against the risk of dyskinesia.
Common Side Effects-and the Science Behind Them
Even with carbidopa’s protective effect, some side effects persist because dopamine’s influence spreads beyond motor pathways:
- Nausea & vomiting: Residual peripheral dopamine still stimulates chemoreceptor trigger zones.
- Orthostatic hypotension: Dopamine dilates peripheral vessels, lowering blood pressure on standing.
- Hallucinations: Excess central dopamine can affect the visual cortex, especially in older patients.
- Motor fluctuations: Long‑term use reshapes receptor sensitivity, causing “wearing‑off” periods.
Many clinicians add a COMT inhibitor a drug that blocks catechol‑O‑methyltransferase, an enzyme that degrades levodopa (e.g., entacapone) or an MAO‑B inhibitor a medication that prevents breakdown of dopamine by monoamine oxidase B (e.g., selegiline) to smooth out plasma levels.
Comparison: Carbidopa‑Levodopa vs Levodopa Alone
| Feature | Carbidopa‑Levodopa | Levodopa Only |
|---|---|---|
| Brain delivery efficiency | ~85% | ~10‑15% |
| Typical dose (mg levodopa) | 250‑500 mg | 500‑1000 mg |
| Peripheral nausea incidence | 15‑20% | 30‑40% |
| Risk of dyskinesia (long‑term) | Similar, dose‑dependent | Higher because of larger doses |
| Compatibility with COMT inhibitors | Yes | Yes, but higher levodopa doses needed |
The numbers make it crystal clear: the combination is the smarter, more patient‑friendly option.
Practical Tips for Patients and Caregivers
- Take on an empty stomach: Wait 30 minutes after a light snack or 1 hour after a full meal.
- Stay consistent: Small timing variations can cause “pump‑up” or “wear‑off” cycles.
- Monitor for dyskinesia: If involuntary movements appear, ask the doctor about dose adjustment or adding a COMT inhibitor.
- Hydrate well: Adequate fluids help maintain blood pressure and reduce constipation, a common side effect of dopaminergic therapy.
- Keep a symptom diary: Note times of onset, peak, and any side effects. This data guides clinicians in fine‑tuning the regimen.
Future Directions: Beyond Carbidopa‑Levodopa
While the combo remains gold‑standard, research is probing new delivery methods-intestinal gels, subcutaneous pumps, and even gene therapy-to bypass oral limitations entirely. Until those become mainstream, understanding the current mechanism is essential for optimizing treatment and setting realistic expectations.
Frequently Asked Questions
Why does carbidopa not cause side effects like levodopa?
Carbidopa is designed not to cross the blood‑brain barrier, so it only blocks peripheral AADC. Because it never reaches the brain, it doesn’t affect central dopamine production, which means it adds no new neurological side effects.
Can I take carbidopa‑levodopa with protein‑rich meals?
High‑protein foods compete for the LNAA transporter, reducing levodopa absorption. It’s best to take the medication at least 30 minutes before or 1 hour after a protein‑heavy meal.
What is the difference between immediate‑release and sustained‑release forms?
Immediate‑release tablets start working within 30‑60 minutes but wear off after 3‑5 hours. Sustained‑release capsules provide a steadier plasma level, extending benefit to 6‑8 hours and reducing the number of daily doses.
Why do some patients develop dyskinesias after years of use?
Long‑term, high‑dose dopamine stimulation can cause the brain’s motor circuits to become hypersensitive, leading to involuntary movements. Adjusting the dose, adding a COMT inhibitor, or using a dopamine agonist can help manage this.



Linda A
October 18, 2025 AT 13:57In the grand theatre of neuropharmacology, the duet of carbidopa and levodopa performs a delicate choreography that restores dopamine's lost rhythm. By holding back peripheral conversion, carbidopa lets levodopa stride confidently across the blood‑brain barrier, where it finally takes the stage as dopamine. The result is a smoother, longer‑lasting encore of motor control for patients.
Ayla Stewart
October 20, 2025 AT 07:37For anyone starting this regimen, the simplest tip is to take the dose on an empty stomach or at least 30 minutes before a protein‑rich meal. This avoids competition for the LNAA transporter and keeps plasma levels more predictable.
Poornima Ganesan
October 21, 2025 AT 16:57The article does a decent job of outlining the basic steps, but it glosses over the clinical nuance that makes the combination truly indispensable. First, the peripheral AADC inhibition isn’t just about nausea; it profoundly reshapes the pharmacodynamic curve, allowing titration with far fewer peaks and troughs. Second, the competition with dietary amino acids is a dynamic process that fluctuates with every meal, not a static rule you can ignore after the first week. Third, long‑term dyskinesia isn’t merely dose‑dependent-it reflects receptor sensitization that can be mitigated by strategic dosing intervals, something the piece barely mentions. Fourth, the role of adjunct therapies like COMT inhibitors or MAO‑B inhibitors is crucial for patients whose “ON” periods begin to wear off prematurely. Finally, the discussion of sustained‑release formulations should have highlighted how the slower absorption profile reduces motor fluctuations more effectively than simply lowering the immediate‑release dose. In short, the mechanistic overview is useful, but clinicians need the deeper pharmacokinetic insights to truly optimize therapy.
Stephanie Zaragoza
October 22, 2025 AT 15:10Indeed, the points you raise merit attention; however, the omission of receptor‑sensitization dynamics is particularly notable, as this phenomenon underlies many of the late‑stage complications observed in chronic therapy. Moreover, when considering adjunctive agents, one must account for their impact on the half‑life of levodopa‑carbidopa, which-as you correctly note-varies significantly between immediate‑release and sustained‑release formulations; this detail, while subtle, influences dosing schedules profoundly.
James Mali
October 23, 2025 AT 18:57It’s a classic combo that still works.
Karla Johnson
October 25, 2025 AT 07:03The interplay between carbidopa and levodopa is a textbook example of pharmacological synergy, and yet many patients remain unaware of the subtle biochemical dance that underpins their daily dose. When levodopa is ingested alone, roughly 85 % of it is metabolized by aromatic L‑amino acid decarboxylase (AADC) in the gut and plasma before it ever reaches the central nervous system, essentially wasting the drug and generating peripheral dopamine that triggers nausea, vomiting, and orthostatic hypotension. Carbidopa, by contrast, is a polar molecule that cannot cross the blood‑brain barrier; its sole mission is to bind to peripheral AADC with high affinity, turning off that enzymatic leak. This blockade extends levodopa’s half‑life from about 60 minutes to upwards of 90–120 minutes, allowing a larger fraction-estimated at 80‑85 %-to survive transport. The surviving levodopa then hitchhikes on the large neutral amino acid (LNAA) transporter, a carrier shared with L‑tyrosine and L‑phenylalanine, which explains why high‑protein meals can competitively inhibit its uptake. Once inside dopaminergic neurons, levodopa meets the same AADC enzyme, now unhindered, and is decarboxylated to dopamine right where it is needed to replenish depleted synaptic stores. The newly formed dopamine binds to D1 and D2 receptors, rebalancing the direct and indirect basal ganglia pathways that regulate smooth, purposeful movement. Because the central conversion is efficient, clinicians can achieve the same motor control with roughly half the levodopa dose that would be required without carbidopa, translating into fewer tablets and lower cost. In addition to dose sparing, the reduction in peripheral dopamine production markedly cuts the incidence of gastrointestinal side effects, a common reason patients discontinue therapy. However, the system is not without flaws; chronic stimulation of dopamine receptors can lead to receptor supersensitivity, manifesting as dyskinesias, especially when dosing escalates to counteract “wearing‑off” periods. To mitigate this, many neurologists add COMT inhibitors such as entacapone or MAO‑B inhibitors like selegiline, which further smooth plasma levodopa peaks and extend “ON” time. It is also essential to counsel patients on timing their medication relative to meals-taking the dose 30 minutes before a light snack or one hour after a protein‑heavy meal can optimize absorption. Finally, individualized titration remains the cornerstone of therapy: start low, increase slowly, and monitor the patient’s motor response as well as any emerging dyskinesia. By understanding each step of this cascade-from oral ingestion, peripheral blockade, blood‑brain barrier crossing, to neuronal conversion-clinicians can fine‑tune treatment plans that maximize benefit while minimizing adverse effects.
Tracy O'Keeffe
October 26, 2025 AT 07:03Wow, look at that endless parade of textbook jargon-so much for "understanding" when half the readers are lost in a sea of acronyms and overly dramatic metaphors. My brain hurts just reading that, and honestly, the real world clinic doesn't need a dissertation every time we prescribe Sinemet.
Norman Adams
October 26, 2025 AT 20:57Oh, absolutely, because patients really love a 20‑sentence lecture on why their pill works; they'd rather be left in the dark than educated, right?
Margaret pope
October 27, 2025 AT 08:03maybe we can find a middle ground and keep it simple for everyone
Emma Williams
October 28, 2025 AT 14:37True its still a go to combo for many patients
Janet Morales
October 29, 2025 AT 10:03Honestly, calling it “classic” downplays the daily struggle of those who battle motor fluctuations and dyskinesias-this isn’t just a nostalgic nod, it’s a lifeline that deserves ongoing innovation and compassion.
Rajesh Singh
October 30, 2025 AT 02:43While the sentiment is passionate, we must also recognize that the combination remains the most evidence‑based option for the majority, and any push for newer, unproven therapies should be tempered with rigorous clinical data.
Albert Fernàndez Chacón
October 31, 2025 AT 17:37Just a heads‑up for newcomers: keep an eye on how your body reacts when you switch between immediate‑release and sustained‑release forms. The timing can feel different, and a small adjustment in the schedule often smooths out those pesky “ON/OFF” swings.
Drew Waggoner
November 1, 2025 AT 07:30Thanks for the practical tip-I'll definitely watch my dosing windows more closely.
Mike Hamilton
November 2, 2025 AT 16:50In the end, the dance of carbidopa and levodopa is a reminder that good medicine often depends on balance-too much or too little can tip the scales, so we must keep listening to each patient as a unique rhythm.